
- Home
- Our Technology
About Bladder Cancer
Bladder cancer is a common urological cancer that has the highest recurrence rate of any cancer malignancy. Bladder cancer is the 10th most common cancer in the world and also ranked 6th for men. There are about 2.7 million bladder cancer patients globally with about 549,000 new cases and 200,000 deaths per year.
Long-term surveillance remains the cornerstone of long-term management for bladder cancer. Invasive cystoscopic examinations and voided urine cytology has been the gold standard modality for over 80 years. However, cystoscopic approach is very burdensome and invasive for patients and costly for healthcare providers; hence there had been a decade-long search for non-invasive urinary biomarkers that can match or even improve upon the sensitivity and specificity of cystoscopy. BioCheetah’s multiplex biomarker panel offers unprecedented accuracy and sensitivity for the diagnosis of bladder cancer patients simply from a few drops of urine.
Our Cancer Biomarker Discovery Technology
Our Research and Development (R&D) laboratory uses a high-resolution, mass spectrometry-based, quantitative proteomics approach, where measurements are compared and validated for protein variations in 451 voided urine samples from a pool of healthy subjects, non-bladder cancer patients and patients with non-invasive and invasive bladder cancer in order to discover novel protein biomarkers present in liquid biopsy samples. Based on this methodology, the identification of five highly sensitive and specific biomarkers were discovered for the diagnosis of bladder cancer1.
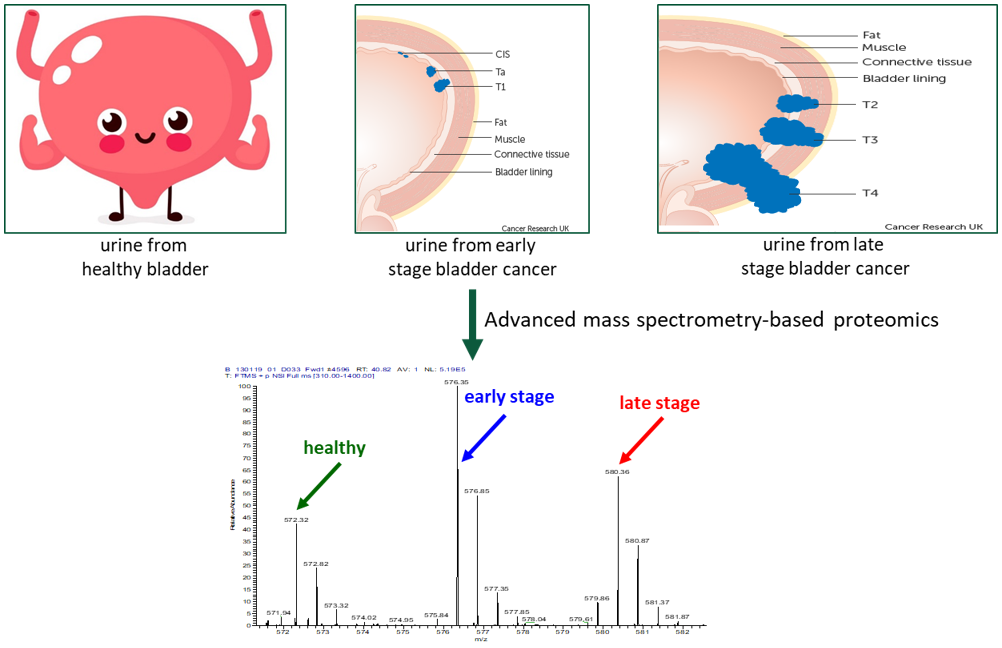
Our Cancer Immunodiagnostic Technology
In order to detect the five biomarkers present in urine samples of bladder cancer patients, rabbit monoclonal antibodies was utilized as it has been recognized as the best in class antibody for diagnostic assays resulting in higher specificity & affinity to their targets.
By combining our IVD products containing antibodies that can detect the five novel urinary biomarkers and our proprietary algorithm, we are able to display outstanding performance in discriminating bladder cancer patients from non-bladder cancer cases within a shorter turnaround time.
A selection of our research publications can be found in these links:
- Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma
- Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors
- c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression.
- FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder.
